That insight was the result of a recent discussion with Mordy Serle, one of the three founders of the Covid Plasma Initiative (CPI), together with Chaim Lebovits and Abba Swiatycki.
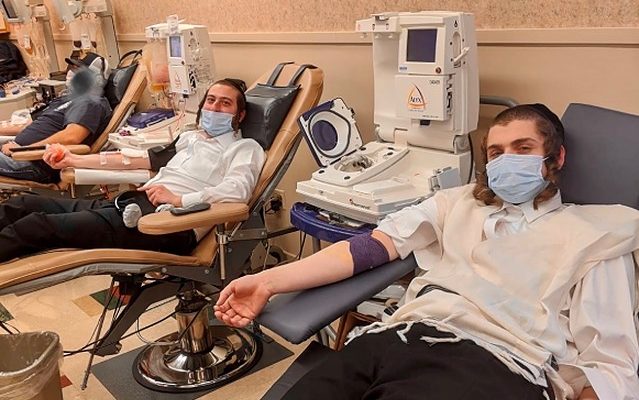
I first "met" Serle nearly a year ago, while writing a Mishpacha feature ("Gift of Life," Issue #807, April 22, 2020) on the Covid Plasma Initiative, a remarkable grassroots project utilizing the blood plasma from recovered COVID-19 patients in the Chareidi community. When Serle reached out to me recently, I assumed it was to update me how the CPI had developed over the past year. But I was wrong.
To be sure, all the dedicated volunteers involved in CPI would have had a great deal to be proud of, had they done nothing more than loose the wellsprings of the Chareidi community's generosity in terms of blood plasma donorship. Every week, 20,000 units of blood plasma from recovered COVID-19 patients are still being shipped around the United States, about 400,000 units in all since the start of the pandemic. Of that figure, some 90,000, over 22 percent of the national total, were contributed by Chareidi Jews. And in the initial months of the use of blood plasma, the percentage contributed by Chareidim was closer to 50 percent.
According to Dr. Michael Joyner of the Mayo Clinic, who was initially charged by the federal government with responsibility for monitoring the efficacy of blood plasma infusions, without the initial outpouring of Chareidi volunteers, it is unlikely that the entire idea of blood plasma infusions would have taken off (though the technique is an old one), or that there would have been enough plasma available to evaluate the efficacy of the treatment or its safety.
And the very public participation of Chareidim in the donations, especially in places far afield from Chareidi population centers, like a blood center in Delaware, attracted a great deal of media attention.
The Wall Street Journal, the New York Times, NBC, CBS, CNN, and Fox News all covered the story at length. And that story triggered what might be called a "virtuous cycle." As news of the potential of blood plasma from recovered COVID-19 sufferers spread, the lines of those eager to donate their blood grew, as did the number of facilities prepared to draw that blood.
Recently, I came to an additional understanding of our Sage's statement, "Mitzvah goreres mitzvah — one mitzvah leads to another" (Pirkei Avos 4:2 ). The pleasure of doing an act of greatness ben adam l'chaveiro that benefits others is so great that one immediately begins looking for additional ways to help.
But Mordy had not contacted me just to provide a follow-up on the blood plasma drives. Rather, he wanted to raise awareness about a new treatment that has given rise to another CPI initiative — the second mitzvah brought about by the first.
Since November, CPI has branched out into a different but related field: synthetically created antibodies, or monoclonal antibodies, produced by two major drug manufacturers, Regernon and Eli Lilly. (Each of the drug companies chose to copy a slightly different antibody.)
Though blood plasma unquestionably saved many thousands of lives, the therapy had one weakness: the units of plasma are not uniform, as the antibody levels produced by recovered patients vary from one to the other. And it turned out that only high titer units — i.e., ones with high antibody levels — were likely to be effective in treatment. Monoclonal antibodies do not have that drawback, as all units are uniform.
The FDA granted emergency use authorization last November for the monoclonal antibodies. That authorization, however, covered only high-risk individuals, either by virtue of age or pre-existing conditions such as obesity, diabetes, high blood pressure, respiratory conditions, or immunosuppressed conditions, which at some infusion centers is read to include pregnancy.
Despite the development of effective COVID-19 vaccines, the need for effective early-stage treatments remains high. New York and New Jersey, two states with high concentrations of Jewish residents, have the second- and third-highest rates of new infections in the country.
CPI had been tracking the development of monoclonal antibodies since prior to their authorization. And its directors realized that the wide web of contacts developed by CPI since the beginning of the pandemic, and the dedicated staff of volunteers (each one of whom holds down a "day job") manning a 24/7 hot-line headed by CEO Zeldy Oppen, could be mobilized for the utilization of the monoclonal antibodies. But first an entirely new set of protocols would have to be developed.
One of the most important lessons learned from the blood plasma project was the importance of providing the plasma as soon as possible after the onset of the coronavirus. And the same is true of the monoclonal antibodies. Though there is some evidence that they help even after hospitalization, the trials upon which the FDA relied were all based on patients who were administered the monoclonal antibodies within four days of being diagnosed.
CPI set itself an even stricter target — to get infusions for those eligible within 24 hours of diagnosis. But that would require people knowing about the treatment even before they are diagnosed with COVID-19. To that end, CPI undertook a massive media campaign, via webinars, social media, billboards — directed both at primary care physicians and the general public.
That campaign encouraged those who showed any symptom of COVID-19 to immediately get tested for the virus. (Testing is readily available in many of New York's religious neighborhoods due to the combined efforts of local physicians, labs, and urgent care centers.)
The next step was to collect all the information about who qualifies for the new therapy, and where infusions can be obtained for those who do qualify. One of the important discoveries CPI made early in the game was that the new therapy is being dramatically underutilized. Too few people and doctors are even aware of its availability. Thus, at a Duke Margolis Health Center conference in February, the chief scientific officer of Eli Lilly noted that of the first million units shipped by the company, only about 25 percent had been used to date.
And that, despite very favorable results from randomized control trials (RCTs). In one such trial, no patient administered the monoclonal antibodies subsequently died, but two percent of those in the control group did. In another trial, in which monoclonal antibodies were used prophylactically in a nursing home where COVID-19 had broken out, four of those in the control group died, but none died among those who received the monoclonal antibodies. (I trust readers will remember the point I have made several times: RCTs raise ethical problems because "unlucky losers" in the placebo group may die as a result of not receiving the potentially life-saving drug.)
The underutilization of the monoclonal antibodies provided an opening for a well-organized community organization like CPI to ensure that the members of the community served were very likely to receive it. The 24/7 hotline run by CPI, under tight rabbinical supervision, guides candidates for the treatment through the process, and makes it much more likely that those who start the process of receiving the infusions see it through to the end.
Mordy estimates that of the 4,000 to 5,000 Jews whom CPI has connected to infusion centers thus far, well under one percent have subsequently been hospitalized, as opposed to the preceding rate for such patients of about 15 percent. But as was the case with the blood plasma drives, the benefits of CPI's expertise have not been confined to the Jewish community.
Chaim Lebovits, the Yiddish-speaking shoe distributor from Monsey, whose wide circle of contacts at New York City's Mount Sinai Hospital and at numerous other metropolitan area hospitals was crucial to the establishment of CPI at the outset of the pandemic, has been working closely with a Hispanic community organization in El Paso, Texas, to guide them in obtaining the monoclonal antibodies for their community. And Mordy Serle spoke at the Duke Margolis Health Center conference on how to navigate the labyrinth of obstacles to obtain the monoclonal antibodies.
CPI has even established adjunct organizations in Canada and Israel. Working together with Eli Lilly–Israel, CPI has encouraged Israel's Ministry of Health, Israeli hospitals, and the health funds to take advantage of the treatment, with indifferent results so far, though the treatment can be obtained in Israel through the CPI adjunct.
B'ezras Hashem, the pandemic will soon wind down. But if not, I fully expect to receive an email from Mordy Serle in about six months raising awareness of another way CPI has found to save lives and make a kiddush Hashem at the same time.
(COMMENT, BELOW)
JWR contributor Jonathan Rosenblum is founder of Jewish Media Resources and a widely-read columnist for the Jerusalem Post's domestic and international editions and for the international glossy, Mishpacha, where this first appeared. He is also a respected commentator on Israeli politics, society, culture and the Israeli legal system, who speaks frequently on these topics in the United States, Europe, and Israel. His articles appear regularly in numerous Jewish periodicals in the United States and Israel. Rosenblum is the author of seven biographies of major modern Jewish figures. He is a graduate of the University of Chicago and Yale Law School. He lives in Jerusalem with his wife and eight children.


 Contact The Editor
Contact The Editor
 Articles By This Author
Articles By This Author